- Isotopes and radioactive decay As mentioned above, isotopes are different forms of an element that have the same number of protons but different numbers of neutrons. Many elements—such as carbon, potassium, and uranium—have multiple naturally occurring isotopes.
- Isotopes: Isotopes are atoms of same elements having same atomic number but different mass numbers. This clearly indicates that isotopes have same number of electrons and protons but different.
Learning Objectives
- Explain what isotopes are and how an isotope affect an element's atomic mass.
- Determine the number of protons, electrons, and neutrons of an element with a given mass number.
It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors.
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. But some carbon atoms have seven or eight neutrons instead of the usual six. Atoms of the same element that differ in their numbers of neutrons are called isotopes. Many isotopes occur naturally. Usually one or two isotopes of an element are the most stable and common. Different isotopes of an element generally have the same physical and chemical properties. That's because they have the same numbers of protons and electrons.
An Example: Hydrogen Isotopes
Hydrogen is an example of an element that has isotopes. Three isotopes of hydrogen are modeled in Figure (PageIndex{1}). Most hydrogen atoms have just one proton and one electron and lack a neutron. These atoms are just called hydrogen. Some hydrogen atoms have one neutron as well. These atoms are the isotope named deuterium. Other hydrogen atoms have two neutrons. These atoms are the isotope named tritium.
For most elements other than hydrogen, isotopes are named for their mass number. For example, carbon atoms with the usual 6 neutrons have a mass number of 12 (6 protons + 6 neutrons = 12), so they are called carbon-12. Carbon atoms with 7 neutrons have atomic mass of 13 (6 protons + 7 neutrons = 13). These atoms are the isotope called carbon-13.
Example (PageIndex{1}): Lithium Isotopes
- What is the atomic number and the mass number of an isotope of lithium containing 3 neutrons.
- What is the atomic number and the mass number of an isotope of lithium containing 4 neutrons?
SOLUTION
A lithium atom contains 3 protons in its nucleus irrespective of the number of neutrons or electrons. Charming mother torrent.
a.
[ begin{align}text{atomic number} = left( text{number of protons} right) &= 3 nonumber left( text{number of neutrons} right) &= 3 nonumberend{align} nonumber ]
[ begin{align} text{mass number} & = left( text{number of protons} right) + left( text{number of neutrons} right) nonumber text{mass number} & = 3 + 3 nonumber &= 6 nonumber end{align}nonumber]
b.
[ begin{align}text{atomic number} = left( text{number of protons} right) &= 3 nonumber left( text{number of neutrons} right) & = 4nonumberend{align}nonumber]
[ begin{align}text{mass number} & = left( text{number of protons} right) + left( text{number of neutrons} right)nonumber text{mass number} & = 3 + 4nonumber &= 7 nonumber end{align}nonumber]
Notice that because the lithium atom always has 3 protons, the atomic number for lithium is always 3. The mass number, however, is 6 in the isotope with 3 neutrons, and 7 in the isotope with 4 neutrons. In nature, only certain isotopes exist. For instance, lithium exists as an isotope with 3 neutrons, and as an isotope with 4 neutrons, but it doesn't exist as an isotope with 2 neutrons or as an isotope with 5 neutrons.
Stability of Isotopes
Atoms need a certain ratio of neutrons to protons to have a stable nucleus. Having too many or too few neutrons relative to protons results in an unstable, or radioactive, nucleus that will sooner or later break down to a more stable form. This process is called radioactive decay. Many isotopes have radioactive nuclei, and these isotopes are referred to as radioisotopes. When they decay, they release particles that may be harmful. This is why radioactive isotopes are dangerous and why working with them requires special suits for protection. The isotope of carbon known as carbon-14 is an example of a radioisotope. In contrast, the carbon isotopes called carbon-12 and carbon-13 are stable.
This whole discussion of isotopes brings us back to Dalton's Atomic Theory. According to Dalton, atoms of a given element are identical. But if atoms of a given element can have different numbers of neutrons, then they can have different masses as well! How did Dalton miss this? It turns out that elements found in nature exist as constant uniform mixtures of their naturally occurring isotopes. In other words, a piece of lithium always contains both types of naturally occurring lithium (the type with 3 neutrons and the type with 4 neutrons). Moreover, it always contains the two in the same relative amounts (or 'relative abundances'). In a chunk of lithium, (93%) will always be lithium with 4 neutrons, while the remaining (7%) will always be lithium with 3 neutrons.
Dalton always experimented with large chunks of an element - chunks that contained all of the naturally occurring isotopes of that element. As a result, when he performed his measurements, he was actually observing the averaged properties of all the different isotopes in the sample. For most of our purposes in chemistry, we will do the same thing and deal with the average mass of the atoms. Luckily, aside from having different masses, most other properties of different isotopes are similar.

There are two main ways in which scientists frequently show the mass number of an atom they are interested in. It is important to note that the mass number is not given on the periodic table. These two ways include writing a nuclear symbol or by giving the name of the element with the mass number written.
To write a nuclear symbol, the mass number is placed at the upper left (superscript) of the chemical symbol and the atomic number is placed at the lower left (subscript) of the symbol. The complete nuclear symbol for helium-4 is drawn below:
The following nuclear symbols are for a nickel nucleus with 31 neutrons and a uranium nucleus with 146 neutrons.
[ce{^{59}_{28}Ni}]
[ ce{ ^{238}_{92}U}]
In the nickel nucleus represented above, the atomic number 28 indicates the nucleus contains 28 protons, and therefore, it must contain 31 neutrons in order to have a mass number of 59. The uranium nucleus has 92 protons as do all uranium nuclei and this particular uranium nucleus has 146 neutrons.
Another way of representing isotopes is by adding a hyphen and the mass number to the chemical name or symbol. Thus the two nuclei would be Nickel-59 or Ni-59 and Uranium-238 or U-238, where 59 and 238 are the mass numbers of the two atoms, respectively. Note that the mass numbers (not the number of neutrons) are given to the side of the name. Oasis whats the story morning glory rar.
Example (PageIndex{2}): POTASSIUM-40
How many protons, electrons, and neutrons are in an atom of (^{40}_{19}ce{K})?
Isotope Symbol Calculator
SOLUTION
[text{atomic number} = left( text{number of protons} right) = 19]

For all atoms with no charge, the number of electrons is equal to the number of protons.
[text{number of electrons} = 19]
The mass number, 40 is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
[text{number of neutrons} = 40 - 19 = 21.]
Example (PageIndex{3}): Zinc-65
How many protons, electrons, and neutrons are in an atom of zinc-65?
SOLUTION
[text{number of protons} = 30]
For all atoms with no charge, the number of electrons is equal to the number of protons.
[text{number of electrons} = 30]
The mass number, 65 is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
[text{number of neutrons} = 65 - 30 = 35]

Exercise (PageIndex{3})
How many protons, electrons, and neutrons are in each atom?
- (^{60}_{27}ce{Co})
- Na-24
- (^{45}_{20}ce{Ca})
- Sr-90
- Answer a:
- 27 protons, 27 electrons, 33 neutrons
- Answer b:
- 11 protons, 11 electrons, 13 neutrons
- Answer c:
- 20 protons, 20 electrons, 25 neutrons
- Answer d:
- 38 protons, 38 electrons, 52 neutrons
Summary
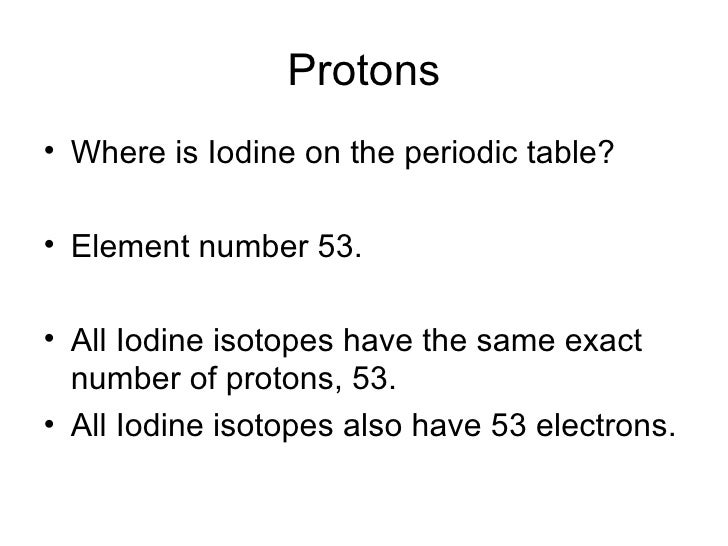
- The number of protons is always the same in atoms of the same element.
- The number of neutrons can be different, even in atoms of the same element.
- Atoms of the same element, containing the same number of protons, but different numbers of neutrons, are known as isotopes.
- Isotopes of any given element all contain the same number of protons, so they have the same atomic number (for example, the atomic number of helium is always 2).
- Isotopes of a given element contain different numbers of neutrons, therefore, different isotopes have different mass numbers.
Contributors
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
Having a basic understanding of isotopes and nuclides is vital to understanding many aspects ofnuclear energy. Here we present a quick and simple review (or preview!) of science class.
Isotopes
Elements are your basic chemical building blocks. They include things like hydrogen, oxygen, sodium,magnesium, iron, titanium…, anything on the periodic table of the elements. Each element on theperiodic table has a different number of protons in its atomic nucleus (its dense center). Eachelement has a few varieties with the same number of protons, but different numbers of neutrons inthe nucleus. All isotopes of a particular element act chemically-identically to each other. Figure 1shows the periodic table of the elements. Each element listed has many (between 2 and 20+) isotopes.
The arrow points to Iron (Fe), which has a few isotopes shown in Figure 2.
Figure 2.The isotopes of Iron
Make sense? Great. One particularly relevant set of isotopes acting chemically similar butneutronically different are those of the element Uranium, shown below in Figure 3.
Enrichment
Now that you know what isotopes are, you can understand exactly what enrichment is.
Natural Uranium is made up of 99.2745% U-238 and 0.720% U-235, with trace amounts of U-234. WhileU-238 usually stays together in a neutron field, U-235 readily splits, or fissions, in the presenceof neutrons, releasing huge amounts of energy. This energy runs nuclear reactors and nuclear weaponsalike. To create a chain reaction, you must enrich natural Uranium to contain more U-235. Atypical nuclear reactor requires about 3.5% U-235, whereas a nuclear weapon requires more than 90%U-235. See figure below.
Enrichment is a very difficult process, as the mass difference between each isotope is minuscule.The US developed enrichment at Oak Ridge during World War II as part of the Manhattan Project. Thenewsworthy item about it is that anyone who can enrich can create highly-enriched uranium, a goodmaterial with which to make nuclear weapons. Countries that want to have their own nuclear fuelmanufacturing capabilities argue that they need enrichment plants, but opponents argue that they arejust looking to produce nuclear weapons.
Isotopes Have Common Atomic Number
Grammar alert: Isotopes vs. Nuclides
Isotope and nuclide are closely related terms. When one speaks of isotopes, they are referring tothe set of nuclides that have the same number of protons. Nuclide is a more general term, referringto a nuclear species that may or may not be isotopes of a single element. Examples:
- “U-235 is my favorite isotope of Uranium.”
- “Cm-244, Pu-241, and Am-242m are lesser known fissile nuclides.”
Isotopes Have The Same Atomic
Many people use them interchangeably, including experts in the field. Just read the MCNP manual! Fl 20 mac.
